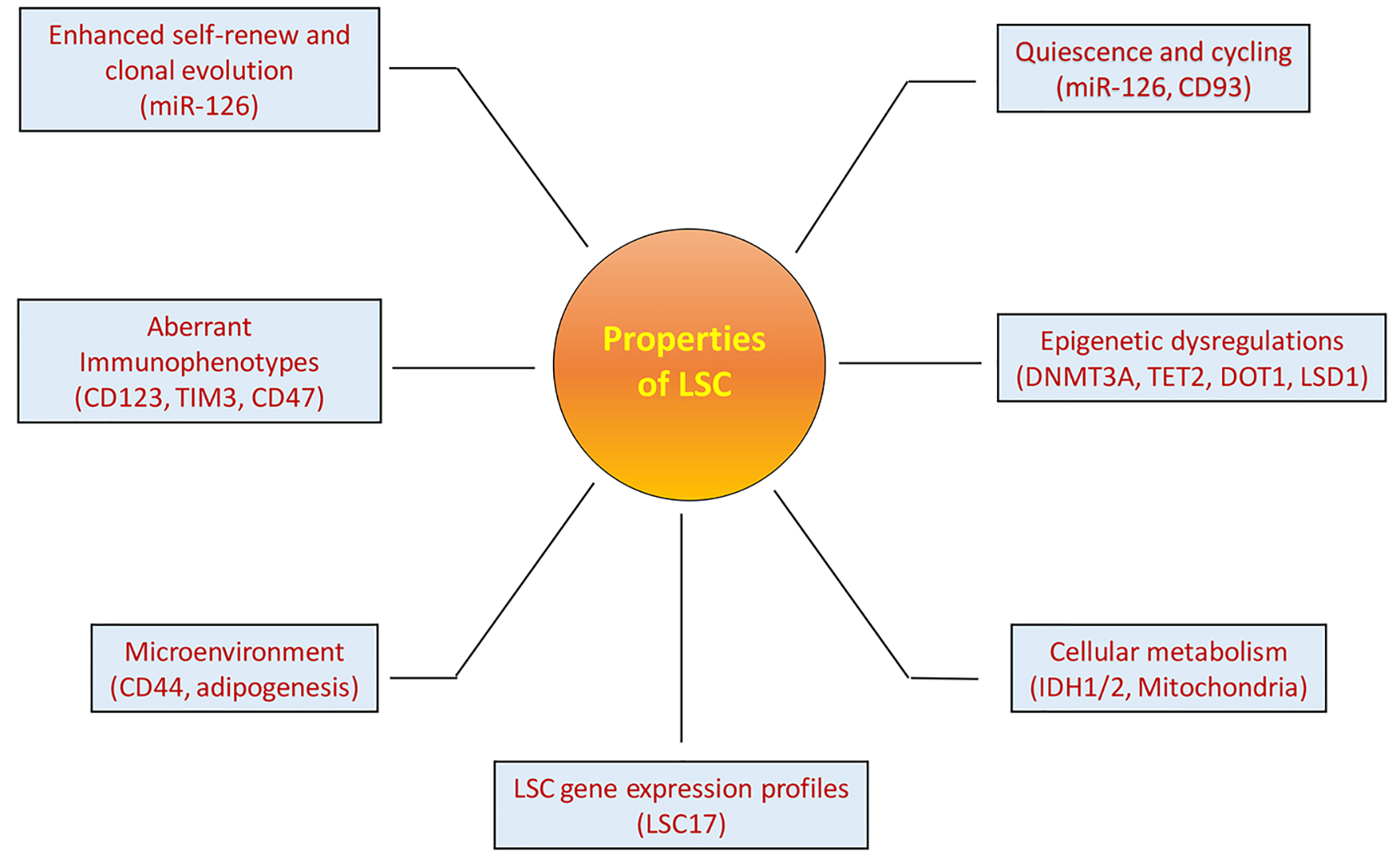
Figure 1. Properties of LSCs. LSCs have multiple specific properties that are important for LSCs to maintain leukemia clones and contribute to leukemia progression and relapse. These properties are also important for identification and targeting of LSCs as discussed in the article. The names in the brackets under each property are the example characteristics of LSCs.
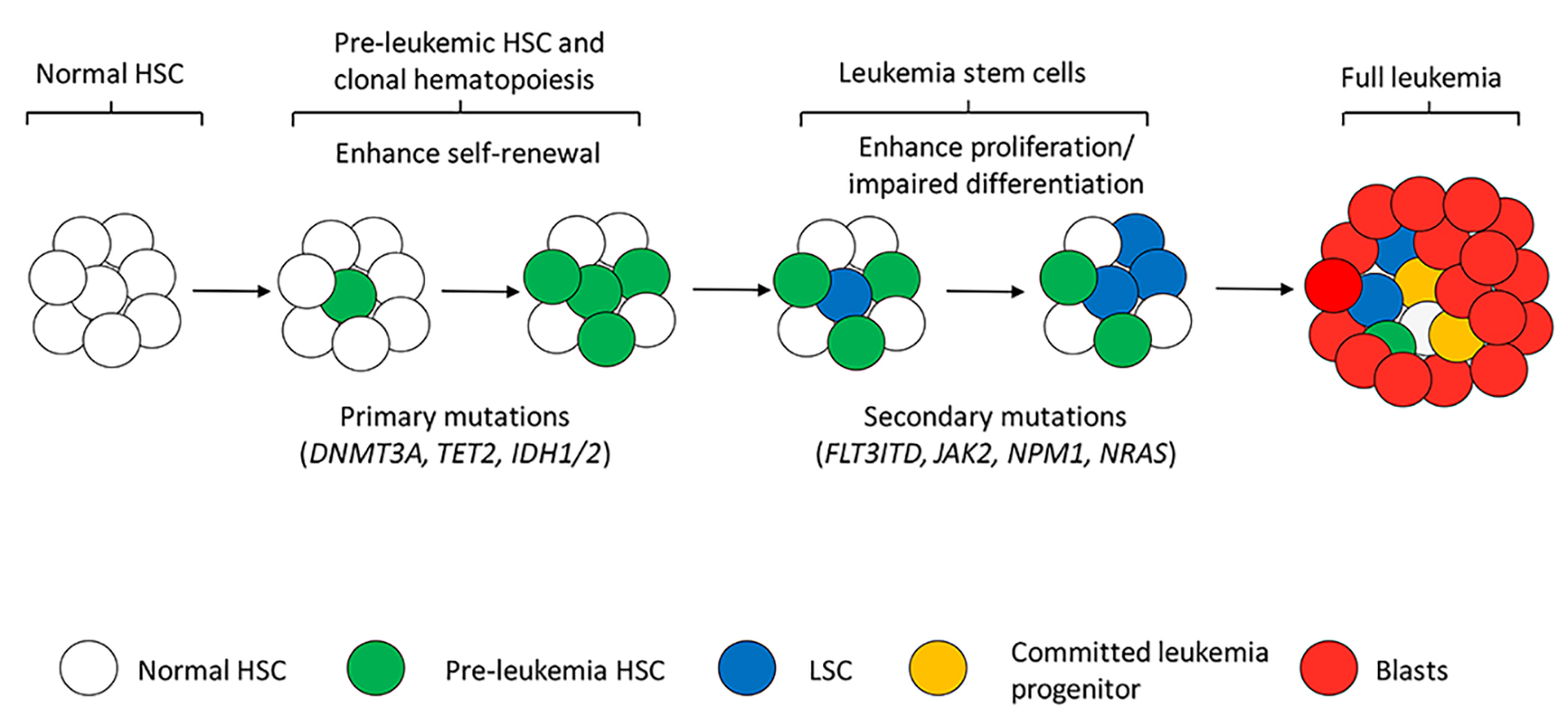
Figure 2. Simplified model of multistep leukemogenesis and leukemia heterogeneity. Acquisition of primary mutations mainly DNMT3A, TET2, IDH1/2 and others modifies NDA methylation status in normal HSCs, resulting in enhanced self-renewal and clonal hematopoiesis (pre-leukemic HSCs). Pre-leukemic HSCs with the primary mutations are responsible only for clonal hematopoiesis or early stage of leukemic process which is not irreversible to leukemia transformation. Acquisition of secondary mutations like FLT3-ITD and JAK2 provides pre-leukemic HSCs the ability of proliferation. Mutated HSCs with both self-renewal and proliferation abilities are considered LSCs that create leukemia clones, produce daughter progenitors and blasts cells. Together distinct types of cells form the complex leukemia heterogeneity. Differentiation programs should also be affected by the genetic alterations in LSCs for accumulation of immature blasts in frank leukemia.
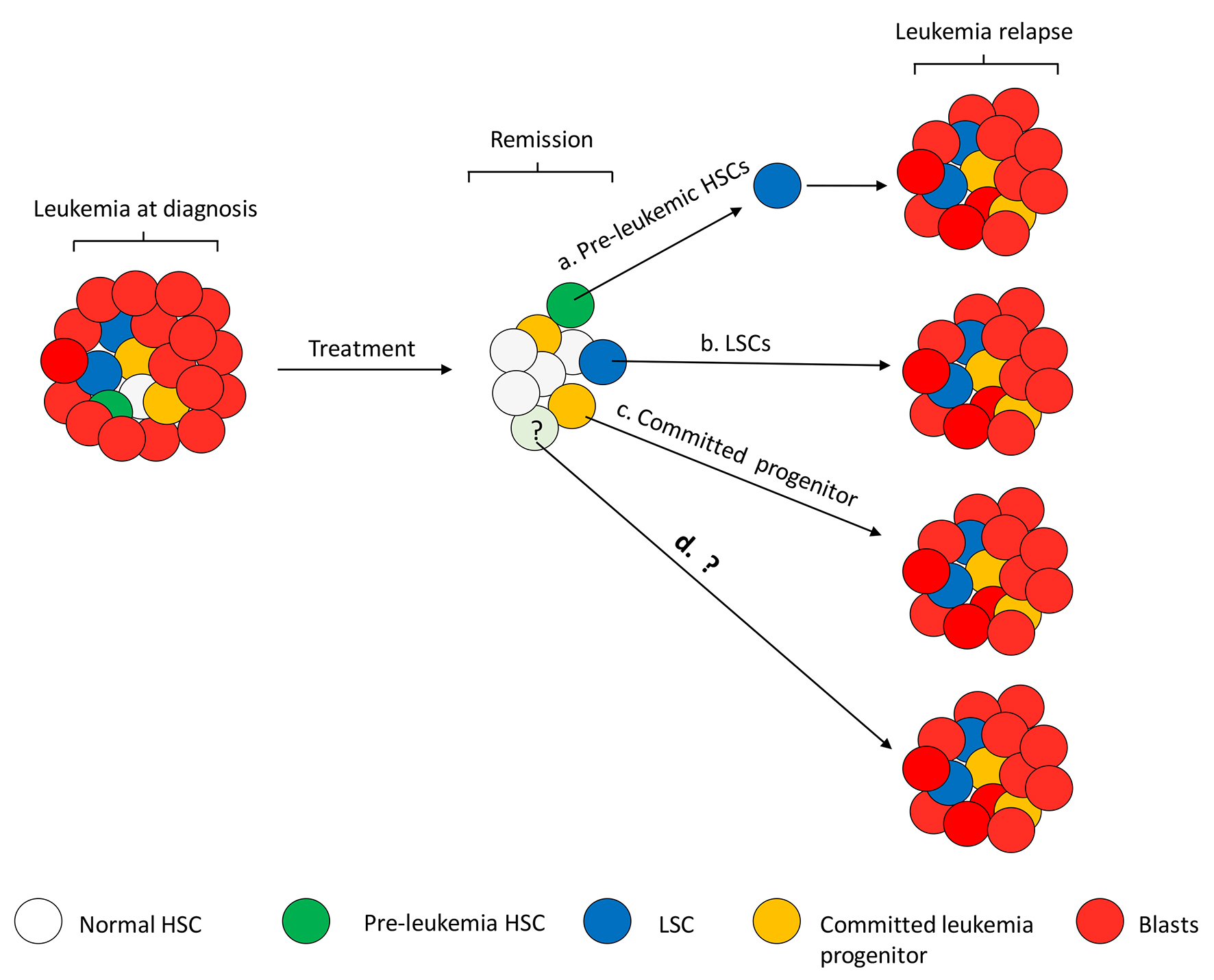
Figure 3. Speculated mechanism of leukemia relapse. Relapse can happen at any time in AML patients with complete remission (CR), from as early as a couple of months to a few or up to 5 years since CR is achieved. Proliferation of different types of residual cells in a patient’s BM may contribute to relapse. To summarize the studies by Shlush et al, relapse could arise from a rare pre-leukemic HSC (e.g. DNMT3A) that needs to acquire additional mutations (e.g. NPM1) to become LSCs and result in relapse (a), or rare dormant LSCs that are resistant to chemotherapies proliferate for leukemia relapse (b). Relapse from rare pre-leukemic HSCs or LSCs takes longer time than from more committed progenitor cells (c). Not all the patients tested in the studies by Shlush et al fit into the categories of cell of origin for relapse, indicating other mechanisms may be also involved in relapse (d).


