| Cellular and Molecular Medicine Research, ISSN 2817-6359 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cell Mol Med Res and Elmer Press Inc |
| Journal website https://www.thecmmr.org |
Original Article
Volume 1, Number 2, December 2023, pages 44-50
Pleiotropic Effects of Improved Apoprotein A-I Mimetic Peptide
Yoshino Matsuoa, Yasunori Suematsua, Hidetaka Moritaa, Emi Kawachib, Takashi Kuwanoa, Satoshi Imaizumia, b, Shin-ichiro Miuraa, c
aDepartment of Cardiology, Fukuoka University School of Medicine, Fukuoka 814-0180, Japan
bClinical Research and Ethics Centre, Fukuoka University School of Medicine, Fukuoka 814-0180, Japan
cCorresponding Author: Shin-ichiro Miura, Department of Cardiology, Fukuoka University School of Medicine, Fukuoka 814-0180, Japan
Manuscript submitted August 30, 2023, accepted September 7, 2023, published online November 3, 2023
Short title: Pleiotropic Effects of Improved ApoA-I Mimetics
doi: https://doi.org/10.14740/cmmr53
| Abstract | ▴Top |
Background: To prevent the onset and progression of atherosclerotic cardiovascular disease, we previously synthesized a mimetic peptide of apolipoprotein A-I (Fukuoka University ApoA-I Mimetic Peptide (FAMP)), which is a major component of high-density lipoprotein (HDL). We reported that FAMP enhanced HDL-induced endothelial tube formation to help counteract cardiac muscle ischemia, and had anti-inflammatory effects or an effect on cholesterol efflux capacity to prevent coronary atherosclerosis.
Methods: We have now synthesized an improved FAMP (i-FAMP) and examined whether its effects are stronger than those of conventional FAMP. First, human coronary artery endothelial cells (HCECs) were used to measure the tube-forming effects of these peptides. In addition, as an anti-inflammatory effect, we also investigated their inhibitory effects on the secretion of monocyte chemotactic protein-1 (MCP-1) from cells. As an anti-apoptotic effect to prevent cardiac muscle ischemia, the inhibitory effect on caspase-3/7 activation was also measured in H9C2 rat cardiomyocyte cells.
Results: HDL promoted HCEC tube formation and suppressed MCP-1 secretion from HCECs and caspase-3/7 activation in H9C2 cells. The tube formation under HDL with i-FAMP treatment was stronger than that under HDL with FAMP. HDL in the presence of FAMP or i-FAMP significantly suppressed MCP-1 secretion compared to HDL in the absence of FAMP or i-FAMP, whereas there were no significant changes in MCP-1 secretion between HDL with FAMP and HDL with i-FAMP treatment. HDL with i-FAMP and FAMP did not enhance the suppression of caspase-3/7 activation by HDL.
Conclusions: HDL affected HCEC tube formation and had anti-inflammatory and anti-apoptotic effects; i-FAMP may or may not enhance these actions. In some cases, these effects were stronger than those of conventional FAMP.
Keywords: High-density lipoprotein; Apolipoprotein A-I mimetic peptide; Tube formation; Anti-inflammation; Anti-apoptotic action
| Introduction | ▴Top |
A low blood level of high-density lipoprotein cholesterol (HDL-C) is a risk factor for atherosclerotic cardiovascular disease (ASCVD) [1]. This risk has been attributed to the ability of HDL to take up cellular cholesterol from the periphery and to mediate the transport of excess cholesterol to the liver (reverse cholesterol transport (RCT)) [2]. Cholesterol efflux capacity has been shown to be a strong inverse predictor of CVD status [3]. Thus, it is important to be able to increase HDL levels and enhance its biochemical function in RCT. Although HDL is a target in the treatment of ASCVD, there are currently only a limited number of therapeutic options to increase and enhance the function of HDL.
However, an exciting new therapeutic strategy, HDL therapy using reconstituted (r)HDL and apolipoprotein (Apo)A-I mimetic peptides, has been developed [4-7]. We have also been studying HDL therapy using rHDL to improve the function of HDL, and to induce an anti-arrhythmogenic effect and prevent left ventricular remodeling [8-10]. ApoA-I mimetic peptides in addition to rHDL have many pleiotropic effects [11], such as anti-oxidant, angiogenic, anti-inflammatory and anti-thrombotic properties, in addition to their ability to enhance RCT. We previously developed an apolipoprotein A-I mimetic peptide (Fukuoka University ApoA-I Mimetic Peptide (FAMP)) and showed that it has an anti-atherosclerotic effect in mice [12, 13]. We also reported that FAMP enhanced HDL-induced endothelial tube formation to prevent cardiac muscle ischemia, and had an anti-inflammatory effect or an effect on cholesterol efflux capacity to prevent coronary atherosclerosis [14-17]. More recently, we developed improved FAMP (i-FAMP), which has an even stronger anti-atherosclerotic effect to prevent the onset and progression of ASCVD [18]. We hypothesized that i-FAMP could also have pleiotropic effects, such as endothelial tube-forming or anti-apoptosis effects to prevent cardiac muscle ischemia and an anti-inflammatory effect, which may be stronger than those of FAMP. Thus, we sought to demonstrate that i-FAMP may be more useful than FAMP for preventing ASCVD in in vitro studies. We analyzed whether i-FAMP has anti-inflammatory and anti-apoptotic effects, whether it induces endothelial tube formation, and whether these effects of i-FAMP are greater than those of FAMP.
| Materials and Methods | ▴Top |
Preparation of FAMP and i-FAMP
FAMP and i-FAMP were synthesized by an Fmoc (N-[9-fluorenyl] methoxycarbonyl)-based solid-phase peptide synthesis using an automated peptide synthesizer (Pioneer and Model 433A, Applied Biosystems, Inc., Waltham, MA) using the standard Fmoc methodology described previously [12].
Measurement of the secretion of monocyte chemotactic protein-1 (MCP-1)
Human coronary endothelial cells (HCECs, Clonetics, San Diego, CA) were cultured and grown in media. In the experiments, HCECs were grown under serum-free conditions for 24 h and incubated with the indicated concentrations of samples for 24 h. Samples were incubated with HDL with and without FAMP or i-FAMP. The secretion of MCP-1 as a marker of inflammation [19] in the medium from HCECs was measured by ELISA kits (R&D Systems, Minneapolis, MN).
HCEC tube formation assay on Matrigel
Matrix gels were purchased from Chemicon International, Inc. (Temecula, CA) [20]. The gels were allowed to polymerize in a 96-well plate as described previously [21]. Briefly, HCECs were seeded and grown under serum-free conditions. In some experiments, cells were cultured in the presence or absence of different kinds of samples. After cells were washed, HCEC tube formation was observed, and we performed a “pixel analysis” of the area of tube formation to calculate the total number of pixels. The number was counted in three different areas and the average value was determined for each sample. The control sample was defined as 100% tube formation, and the % increase or decrease in tube formation relative to the control was calculated for each sample.
Measurement of caspase-3/7 activities under various treatments in H9C2 rat cardiomyocyte cells
As an anti-apoptotic effect to prevent cardiac muscle ischemia, the inhibitory effect on caspase-3/7 activation was also measured in H9C2 rat cardiomyocyte cells. Briefly, H9C2 cells were seeded and grown under serum-free conditions in the presence or absence of the indicated concentrations of doxorubicin. In some experiments, cells were cultured in the presence or absence of different kinds of samples. Caspase-3/7 activities were measured by a luminescent assay using the Caspase-Glo® 3/7 assay kit (Promega Corp., Madison, WI). Following caspase cleavage of the proluciferin substrate, a substrate for luciferase is released, which results in the luciferase reaction and the production of light. The luminescence of each sample was measured using a plate-reading luminometer.
Statistical analysis
Statistical analysis was performed using the Stat View statistical software package (Stat View 5; SAS Institute INC., Cary, NC). Data are expressed as the mean ± standard deviation (SD). The significance of differences between mean values was evaluated by an unpaired t-test or one-way analysis of variance followed by Fisher’s protected-least-significant-difference test, as appropriate. Statistical significance was set at < 0.05.
| Results | ▴Top |
Effects of HDL in the absence or presence of the indicated concentrations of FAMP or i-FAMP on HCEC tube formation
Fifty µg/µL of HDL, but not FAMP or i-FAMP, significantly induced HCEC tube formation (Fig. 1A). HDL in the presence of FAMP or i-FAMP significantly induced tube formation compared to HDL alone (Fig. 1B). In addition, tube formation with HDL in the presence of i-FAMP was significantly greater than that with HDL in the presence of FAMP.
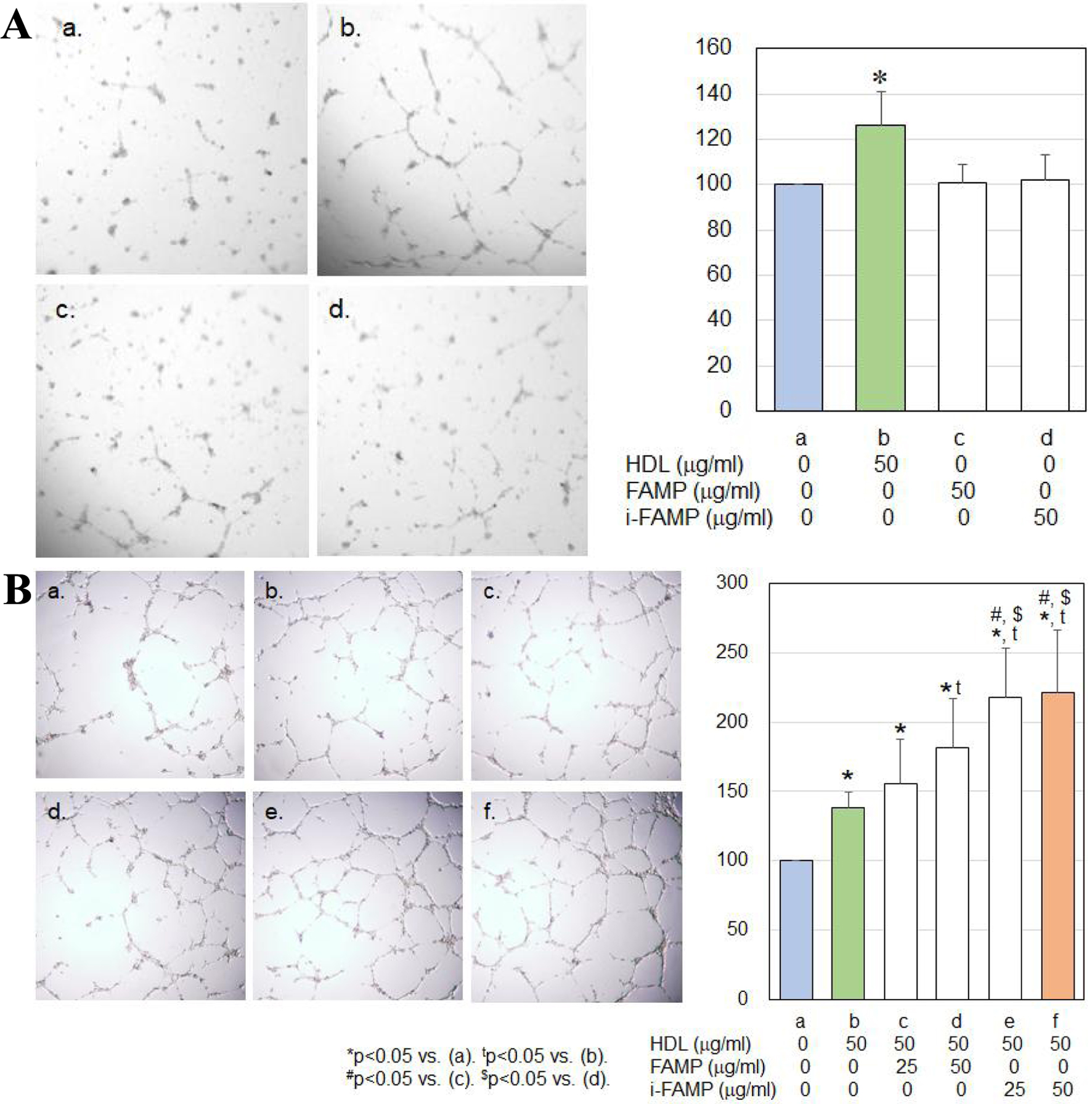 Click for large image | Figure 1. (A) Effects of HDL on HCEC tube formation. (B) Effects of HDL in the absence or presence of the indicated concentrations of FAMP or i-FAMP on HCEC tube formation. HDL: high-density lipoprotein; HCECs: human coronary artery endothelial cells; FAMP: Fukuoka University ApoA-I Mimetic Peptide; i-FAMP: improved FAMP. |
MCP-1 secretion under various treatments in HCECs
There were no significant changes in MCP-1 secretion under treatment with HDL, FAMP or i-FAMP (Fig. 2). HDL in the presence of FAMP or i-FAMP significantly suppressed MCP-1 secretion compared to that with HDL in the absence of FAMP or i-FAMP, whereas there was no significant difference in MCP-1 secretion between HDL with FAMP and HDL with i-FAMP.
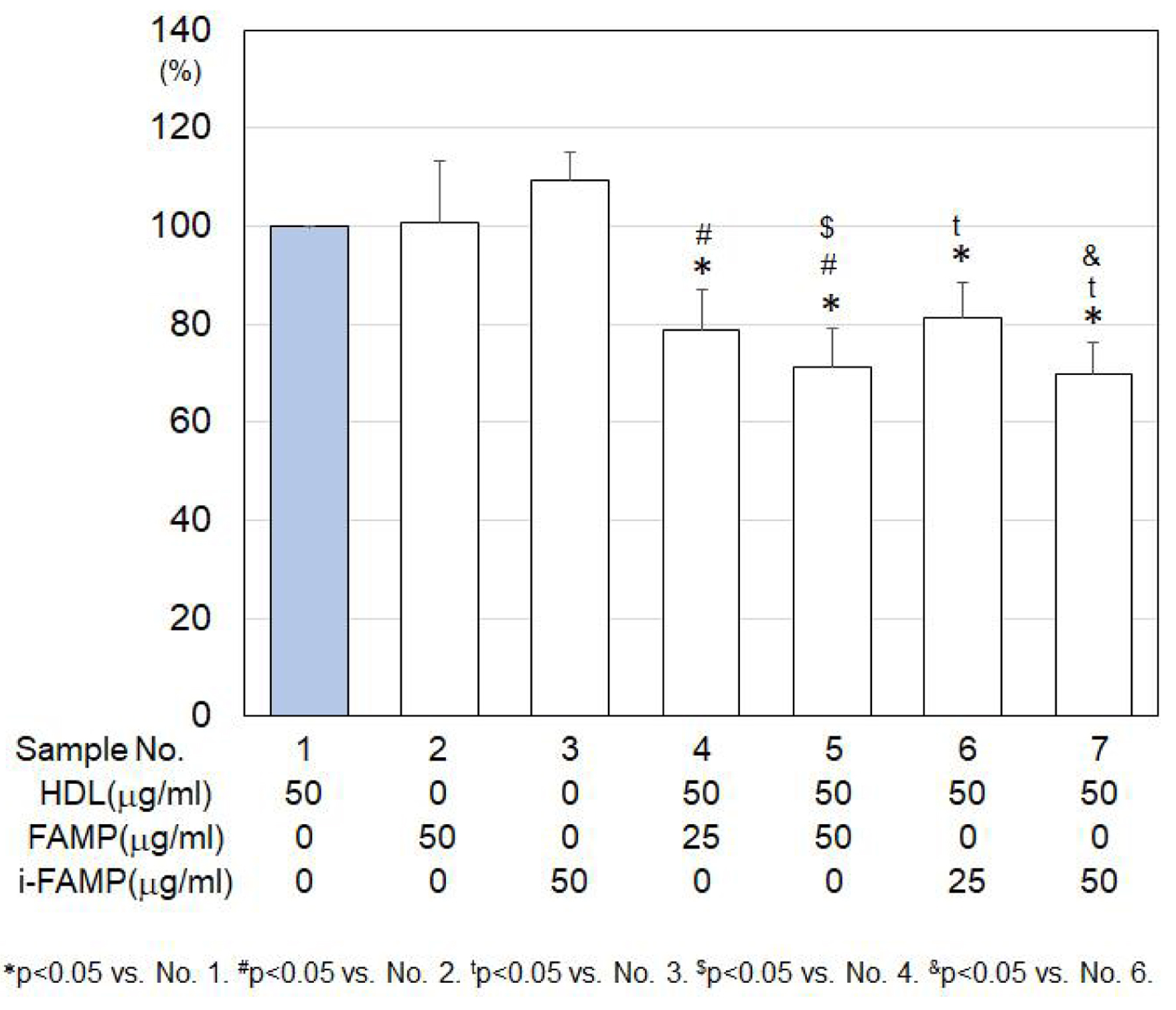 Click for large image | Figure 2. MCP-1 secretion under various treatments in HCECs. MCP-1: monocyte chemotactic protein-1; HCECs: human coronary artery endothelial cells. |
Caspase-3/7 activities using HDL and/or doxorubicin in H9C2 cells
Doxorubicin induced caspase-3/7 activities in a dose-dependent manner (Fig. 3A), and these activities were suppressed by HDL in a dose-dependent manner. Neither HDL with i-FAMP nor HDL with FAMP enhanced the suppression of caspase-3/7 activation by HDL (Fig. 3B).
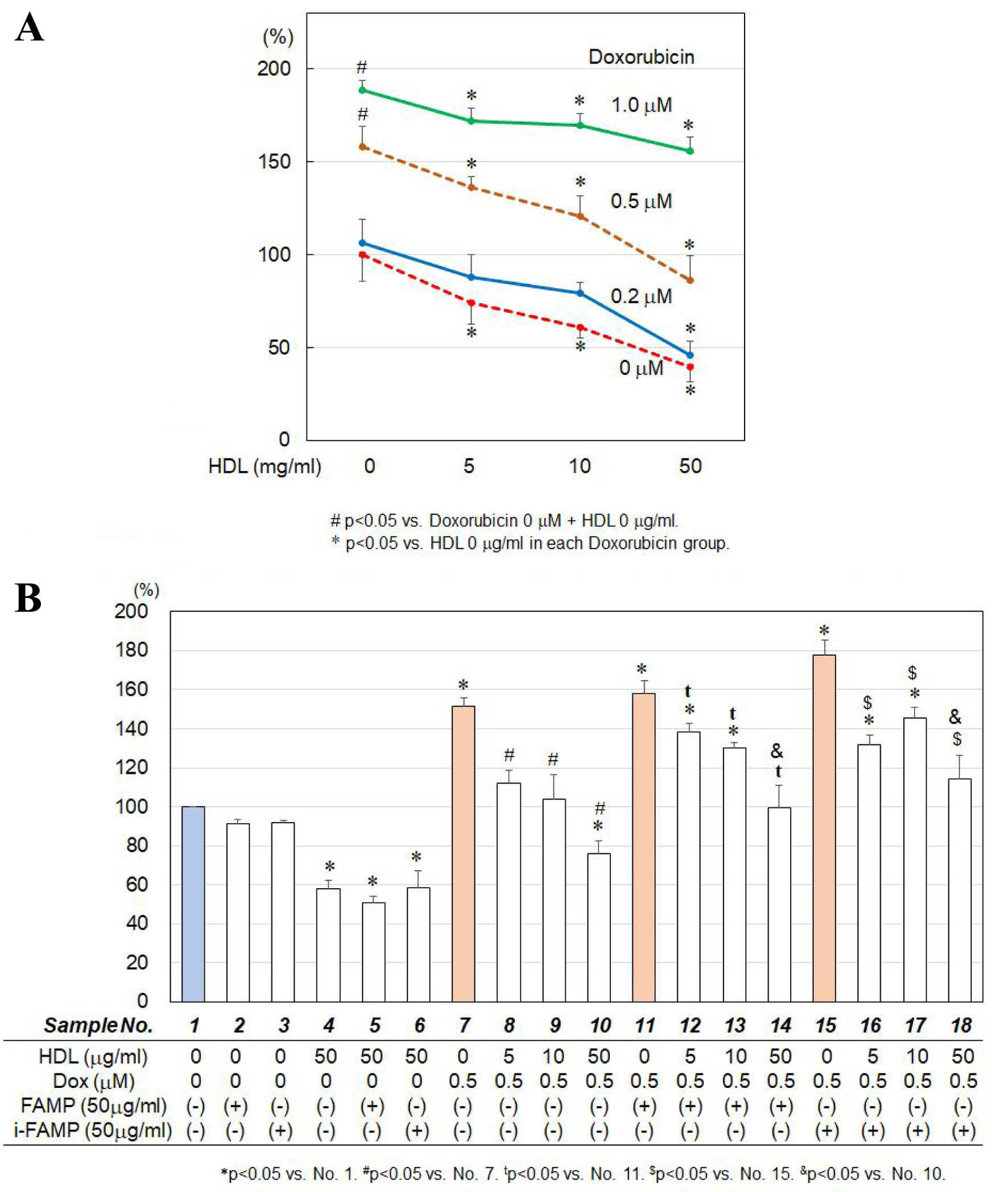 Click for large image | Figure 3. (A) Effects of HDL on doxorubicin-induced caspase-3/7 activities in H9C2 cells. (B) Effects of HDL in the absence or presence of the indicated concentrations of FAMP or i-FAMP on doxorubicin-induced caspase-3/7 activities in H9C2 cells. HDL: high-density lipoprotein; FAMP: Fukuoka University ApoA-I Mimetic Peptide; i-FAMP: improved FAMP. |
| Discussion | ▴Top |
The main finding of the present study is that i-FAMP with HDL has beneficial anti-inflammatory effects and induces HCEC tube formation, although the effect on tube formation, but not its anti-inflammatory effect, was stronger than that of FAMP with HDL. This beneficial effect induced by i-FAMP is very important because one of the major therapeutic goals of modern cardiology is to prevent ASCVD.
Angiogenesis, the process of postnatal neovascularization, is a critical component of several human diseases, including ASCVD, cancer, diabetic microvascular disease and rheumatoid arthritis [22]. i-FAMP-induced HCEC tube formation is a physiological effect that is useful for preventing cardiac muscle ischemia. On the other hand, angiogenesis-induced atherosclerosis is a pathological condition that is associated with the secretion of various inflammatory substances, such as interleukin-6 and MCP-1. FAMP and iFAMP, at least in part, decreased MCP-1 secretion from the cells. In fact, we previously reported that HDL, rHDL and FAMP promoted EC tube formation [14, 22, 23], and this effect may be beneficial in the setting of ischemic diseases, such as ASCVD and peripheral artery disease. In fact, we have reported that HDL, rHDL and FAMP promoted EC tube formation [14, 22, 23], and this effect may be beneficial for ischemic diseases, such as ASCVD and peripheral artery disease. In addition, HDL from healthy subjects enhanced endothelial progenitor cell-mediated tubulogenesis compared to that from donors with CAD [24]. Cell elongation is a key cellular mechanism that promotes angiogenesis. In the present study, HDL with i-FAMP promoted tube formation like cell elongation compared to HDL with FAMP. HDL with i-FAMP therapy might be more useful in patients with ischemic diseases than HDL with FAMP. i-FAMP had a greater effect on tube formation than FAMP, as we hypothesized.
Although we previously reported that i-FAMP significantly suppresses the formation of aortic plaque by enhancing HDL function in a mouse model [18], we needed to analyze the direct role of i-FAMP in this anti-inflammatory effect independent of its ability to enhance reverse cholesterol transport because inflammation plays a pathological role in the progression of atherosclerosis [20]. Although FAMP did not directly decrease the secretion of MCP-1, HDL with i-FAMP significantly decreased the secretion of MCP-1 compared to that seen with HDL without i-FAMP. Since i-FAMP accelerated the generation of pre-β HDL, which is converted from mature HDL [18], and since Troutt et al reported that D-4F induced pre-β1 HDL formation in vitro in human plasma and in mice [25], pre-β HDL may have a stronger anti-inflammatory effect. In addition, the mechanism of action of ApoA-I mimetic peptides has been shown to be due to the remarkable affinity of pro-inflammatory oxidized lipids for these peptides compared with ApoA-I [26]. Although we did not perform a human study using FAMP, an index of HDL inflammation was significantly improved in participants who received D-4F compared with placebo [24]. Unfortunately, there was no significant difference in MCP-1 secretion between HDL with FAMP and HDL with i-FAMP. On the other hand, i-FAMP had a stronger anti-atherosclerotic effect [18]. Since inflammation plays a pathological role in the progression of atherosclerosis, we expected that the anti-inflammatory effect of HDL with i-FAMP should be stronger than that of HDL with FAMP. If we analyze other markers of inflammation, we might find a difference between the abilities of i-FAMP and FAMP. The anti-inflammatory effects of i-FAMP and FAMP may not be related to the difference in their anti-atherogenic effects.
Cardio-oncology or onco-cardiology seeks solutions to address these unmet medical needs [27]. Cardiovascular complications caused by cancer therapy include cancer therapy-related cardiac dysfunction (CTRCD). The anthracycline antibiotic doxorubicin is used in chemotherapy for hematopoietic tumors, and is cardiotoxic in a dose-dependent manner [28]. Although doxorubicin-induced caspase-3/7 activities were suppressed by HDL alone, HDL with either i-FAMP or FAMP did not enhance this suppression of activation by HDL. Several reports have indicated the mechanisms by which HDL exerts an anti-apoptotic effect [29-31]. Treatment with homocysteine significantly increased caspase-3 activity whereas HDL significantly decreased it, compared to the homocysteine-only group [29]. HDL also prevents apoptosis in endothelial progenitor cells through the inhibition of caspase-3 activity. HDL3 antagonizes ox-LDL-induced apoptosis in RAW264.7 cells by reducing the accumulation of toxic cholesterol [30]. In addition, palmitic acid induced the accumulation of reactive oxygen species which resulted in cardiomyocyte apoptosis and inflammation [31]. HDL attenuated palmitic acid-induced lipotoxicity and oxidative dysfunction via the suppression of reactive oxygen species. Thus, HDL with either i-FAMP or FAMP may not enhance these signals compared to HDL alone.
Conclusions
HDL promoted HCEC tube formation and had anti-inflammatory and anti-apoptotic effects, whereas i-FAMP may or may not enhance these effects. In some cases, these effects are stronger than those with FAMP.
Acknowledgments
We thank all laboratory members at the Department of Cardiology, Fukuoka University School of Medicine.
Financial Disclosure
We have no financial disclosure or funding.
Conflict of Interest
We have no conflict of interest to declare.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: YS and SI. Methodology: YM, HM and EK. Validation: TK and SM. Formal analysis: YM. Investigation: YM. Writing original draft preparation: YM. Writing review and editing: SM. Visualization: SI. Supervision: SM. All authors have read and agreed to the published version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Gordon DJ, Rifkind BM. High-density lipoprotein—the clinical implications of recent studies. N Engl J Med. 1989;321(19):1311-1316.
doi pubmed - von Eckardstein A, Nofer JR, Assmann G. Acceleration of reverse cholesterol transport. Curr Opin Cardiol. 2000;15(5):348-354.
doi pubmed - Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127-135.
doi pubmed pmc - Iwata A, Miura S, Zhang B, Imaizumi S, Uehara Y, Shiomi M, Saku K. Antiatherogenic effects of newly developed apolipoprotein A-I mimetic peptide/phospholipid complexes against aortic plaque burden in Watanabe-heritable hyperlipidemic rabbits. Atherosclerosis. 2011;218(2):300-307.
doi pubmed - Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290(17):2292-2300.
doi pubmed - Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, et al. Oral administration of an Apo A-I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002;105(3):290-292.
doi pubmed - Li X, Chyu KY, Faria Neto JR, Yano J, Nathwani N, Ferreira C, Dimayuga PC, et al. Differential effects of apolipoprotein A-I-mimetic peptide on evolving and established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;110(12):1701-1705.
doi pubmed - Imaizumi S, Miura S, Nakamura K, Kiya Y, Uehara Y, Zhang B, Matsuo Y, et al. Antiarrhythmogenic effect of reconstituted high-density lipoprotein against ischemia/reperfusion in rats. J Am Coll Cardiol. 2008;51(16):1604-1612.
doi pubmed - Kiya Y, Miura S, Imaizumi S, Uehara Y, Matsuo Y, Abe S, Jimi S, et al. Reconstituted high-density lipoprotein attenuates postinfarction left ventricular remodeling in rats. Atherosclerosis. 2009;203(1):137-144.
doi pubmed - Imaizumi S, Kiya Y, Miura S, Zhang B, Matsuo Y, Uehara Y, Rye KA, et al. Pharmacological intervention using reconstituted high-density lipoprotein changes in the lipid profile in spontaneously hypersensitive rats. Clin Exp Hypertens. 2010;32(3):202-208.
doi pubmed - Linsel-Nitschke P, Tall AR. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev Drug Discov. 2005;4(3):193-205.
doi pubmed - Uehara Y, Ando S, Yahiro E, Oniki K, Ayaori M, Abe S, Kawachi E, et al. FAMP, a novel apoA-I mimetic peptide, suppresses aortic plaque formation through promotion of biological HDL function in ApoE-deficient mice. J Am Heart Assoc. 2013;2(3):e000048.
doi pubmed pmc - Shimizu T, Tanigawa H, Miura S, Kuwano T, Takata K, Suematsu Y, Imaizumi S, et al. Newly developed apolipoprotein A-I mimetic peptide promotes macrophage reverse cholesterol transport in vivo. Int J Cardiol. 2015;192:82-88.
doi pubmed - Miura S, Suematsu Y, Matsuo Y, Imaizumi S, Yahiro E, Uehara Y, Saku K. Induction of endothelial tube formation and anti-inflammation by newly developed apolipoprotein A-I mimetic peptide. IJC Metabolic & Endocrine. 2014;5:70-72
- Takata K, Imaizumi S, Kawachi E, Yahiro E, Suematsu Y, Shimizu T, Abe S, et al. The ApoA-I mimetic peptide FAMP promotes recovery from hindlimb ischemia through a nitric oxide (NO)-related pathway. Int J Cardiol. 2016;207:317-325.
doi pubmed - Suematsu Y, Miura S, Takata K, Shimizu T, Kuwano T, Imaizumi S, Matsuo Y, et al. A novel inducible cholesterol efflux peptide, FAMP, protects against myocardial ischemia reperfusion injury through a nitric oxide pathway. Int J Cardiol. 2016;202:810-816.
doi pubmed - Yahiro E, Uehara Y, Kawachi E, Ando S, Miura SI, Saku K. Improved survival rate after myocardial infarction using an inducible cholesterol efflux (iCE) peptide: FAMP. Int J Cardiol Heart Vessel. 2014;4:135-137.
doi pubmed pmc - Suematsu Y, Kawachi E, Idemoto Y, Matsuo Y, Kuwano T, Kitajima K, Imaizumi S, et al. Anti-atherosclerotic effects of an improved apolipoprotein A-I mimetic peptide. Int J Cardiol. 2019;297:111-117.
doi pubmed - Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045-2051.
doi pubmed pmc - Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25(2):312-318.
doi pubmed - Miura S, Fujino M, Matsuo Y, Kawamura A, Tanigawa H, Nishikawa H, Saku K. High density lipoprotein-induced angiogenesis requires the activation of Ras/MAP kinase in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23(5):802-808.
doi pubmed - Scheinowitz M. Therapeutic myocardial angiogenesis: past, present and future. Mol Cell Biochem. 2004;264(1-2):75-83.
doi pubmed - Sumi M, Sata M, Miura S, Rye KA, Toya N, Kanaoka Y, Yanaga K, et al. Reconstituted high-density lipoprotein stimulates differentiation of endothelial progenitor cells and enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27(4):813-818.
doi pubmed - Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49(6):1344-1352.
doi pubmed pmc - Troutt JS, Alborn WE, Mosior MK, Dai J, Murphy AT, Beyer TP, Zhang Y, et al. An apolipoprotein A-I mimetic dose-dependently increases the formation of prebeta1 HDL in human plasma. J Lipid Res. 2008;49(3):581-587.
doi pubmed - Van Lenten BJ, Wagner AC, Jung CL, Ruchala P, Waring AJ, Lehrer RI, Watson AD, et al. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J Lipid Res. 2008;49(11):2302-2311.
doi pubmed pmc - Kadowaki H, Akazawa H, Ishida J, Komuro I. Cancer Therapeutics-Related Cardiac Dysfunction - Insights From Bench and Bedside of Onco-Cardiology. Circ J. 2020;84(9):1446-1453.
doi pubmed - Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231-2247.
doi pubmed - Noor R, Shuaib U, Wang CX, Todd K, Ghani U, Schwindt B, Shuaib A. High-density lipoprotein cholesterol regulates endothelial progenitor cells by increasing eNOS and preventing apoptosis. Atherosclerosis. 2007;192(1):92-99.
doi pubmed - Jiang P, Yan PK, Chen JX, Zhu BY, Lei XY, Yin WD, Liao DF. High density lipoprotein 3 inhibits oxidized low density lipoprotein-induced apoptosis via promoting cholesterol efflux in RAW264.7 cells. Acta Pharmacol Sin. 2006;27(2):151-157.
doi pubmed - Wu KM, Hsu YM, Ying MC, Tsai FJ, Tsai CH, Chung JG, Yang JS, et al. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutr Metab (Lond). 2019;16:36.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cellular and Molecular Medicine Research is published by Elmer Press Inc.



