| Cellular and Molecular Medicine Research, ISSN 2817-6359 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cell Mol Med Res and Elmer Press Inc |
| Journal website https://www.thecmmr.org |
Original Article
Volume 1, Number 2, December 2023, pages 51-55
Immunolocalization of Nestin-Positive Cells in Islets of WNIN-Obese Rats: Implication in Obesity and Acute Pancreatitis
Himadri Singha, Vijayalakshmi Venkatesana, b
aDepartment of Biochemistry/Stem Cell Research, National Institute of Nutrition (ICMR), Hyderabad 500007, India
bCorresponding Author: Vijayalakshmi Venkatesan, Department of Biochemistry/Stem Cell Research, National Institute of Nutrition (ICMR), Tarnaka, Hyderabad 500007, India
Manuscript submitted June 13, 2023, accepted August 15, 2023, published online November 7, 2023
Short title: Nestin-Positive Cells in WNIN-Obese Rats
doi: https://doi.org/10.14740/cmmr40
| Abstract | ▴Top |
Background: WNIN-obese rats are novel animal model to study metabolic syndrome. The islets of WNIN-obese rats have increased pancreatic inflammation and stress. The role of nestin in obesity and acute pancreatitis is not clear.
Methods: Our present study demonstrated presence of nestin in the islets of WNIN-obese rats as well as lean counterpart, when induced with acute pancreatitis.
Results: Interestingly, nestin was localized in islets of WNIN-obese rats, but was absent in islets of control rats.
Conclusion: The localization of nestin may have functional significance in obesity and acute pancreatitis as the nestin-positive stem cells may lead to islet adaptation in pancreatic stress and inflammation.
Keywords: Islets; WNIN-obese rats; Nestin; Animal model
| Introduction | ▴Top |
Obesity is characterized by low grade inflammation, leading to cardiovascular diseases, hypertension, and type 2 diabetes [1]. Interestingly, WNIN/Gr-Ob rat strain (herein referred as WNIN-obese rats) is among animal models that have greatly helped our understanding of the pathophysiology associated with obesity. This rat strain is developed from the Wistar rat strain of our institute (herein referred as lean rats), maintained at our institute. These rats portray features of obesity and insulin resistance with distinct clinical as well as biochemical features like hyperinsulinemia, hypertriglyceridemia, and hypercholesterolemia along with hyperphagia, polydipsia, polyuria, and proteinuria similar to other animal models. A recent study has localized the observed unilocus mutation in a 4.3 cm region with flanking markers D5Rat256 and D5Wox37 on chromosome 5 upstream of the leptin receptor [2]. WNIN-obese rats also display reduced lifespan as they show features of degenerative diseases once they cross 1 year. The life span of WNIN-obese rats is 1.5 year as compared to 3 years in lean rats [3]. Increased macro-molecular damage and oxidative stress were observed in neocortex and hippocampus of WNIN-obese rats [4]. Increased cataract development was also observed in WNIN-obese rats due to accumulation of intralenticular sorbitol [5]. Moreover, numerous studies have demonstrated presence of pro-inflammatory milieu in adipose tissue, mesenchymal stem cells and pancreas of WNIN-obese rats [3, 6-9].
Nestin is a marker of neural stem cells or progenitors [10]. Cells expressing nestin were reported to differentiate in vitro into pancreatic endocrine, exocrine, and hepatic phenotypes [11]. Differentiation of islet cells from mouse embryonic stem cell (ESC) is also known to involve an intermediate cell type expressing nestin [12]. However, descriptive analyses of mouse and human development argued against a role of nestin-positive cells in islet differentiation [13, 14]. It is also understood that enriched nestin-positive cells express the ABCG2 and MDR-1 transporters, showing that these cells have properties that are consistent with a multipotent stem cell like population [15]. Further, clinical and molecular analyses have revealed nestin-positive cells having progenitor or stem cell properties [16].
In addition, presence of nestin-positive cells in bone marrow, ESCs and islets may also have functional significance [17]. Nestin-positive cells can be differentiated to islet endocrine cells and transplanted into donors [11]. These cells might serve as precursors of differentiated pancreatic endocrine cells during physiological stress (pregnancy, partial pancreatectomy and obesity) [18]. Numerous studies have demonstrated proliferation of intra-islet progenitors under stress conditions [19]. Interestingly, it has been observed that nestin becomes up-regulated as a response to pancreatic injury [20].
The present investigation is undertaken to assess the presence of nestin-positive cells in islets of WNIN-obese rats and lean rats at basal condition and when acute pancreatic was induced in these rats. As the characteristics and variation in the islet of Langerhans both at basal condition and when induced with pancreatitis are already characterized in our earlier studies [8, 9]. Hence, localization of nestin will help us understand the relation between nestin and islet microenvironment in obesity and acute pancreatitis.
| Materials and Methods | ▴Top |
Experimental animals
Animal experiments were approved by the ethical committee on animal experiments at National Institute of Nutrition, Hyderabad, India (Regd. No. 154/1999/CPCSEA). The experiments were performed in compliance with “principles of laboratory animal care” (NIH publication no. 85-23, revised 1985). WNIN/GR-Ob rats (4 months; referred as WNIN-obese rats) and WNIN Wistar rats (referred as lean rats) were obtained from the National Center for Laboratory Animal Sciences (NCLAS), Hyderabad, India. WNIN-obese rats as well as lean rats were randomly allocated into four groups (six rats in each group): group 1: lean rats treated with saline; group 2: lean rats treated with L-arginine; group 3: WNIN-obese rats administered with saline; and group 4: WNIN-obese rats treated with L-arginine. Acute pancreatic was induced by intra-peritoneal administration of L-arginine hydrochloride as described earlier [21].
Immunolocalization of nestin
Formaldehyde fixed-paraffin embedded sections of the head region of pancreatic tissue were processed for immunolocalization as described earlier [17]. Briefly, after deparaffinization, the sections were dehydrated by passing through a series of decreasing concentration of ethanol. Permeabilization was done using 50% chilled methanol (v/v in water), and the sections were incubated at 37 °C for 1 h using 4% serum for the non-specific blocking. This was followed by overnight incubation with primary antibodies for nestin (BD Biosciences, USA) and insulin (Sigma, St. Louis, MO, USA) at a dilution of 1:100 in a humified chamber. After washes to remove excess unbound antibodies, samples were then incubated with the corresponding anti-rabbit or anti-goat secondary antibodies (anti-rabbit IgG-CFL 488; Santa Cruz Biotechnology Inc.; goat anti-mouse alexa 633, Moleculae Probes, USA). The fluorescent images were visualized on Leica TCS SP5 series confocal microscope.
| Results and Discussion | ▴Top |
Our present study has demonstrated first time, presence of nestin-positive cells in islets of WNIN-obese rats. Interestingly, the induction of pancreatitis led to increased expression of nestin in these cells. These nestin-positive cells were localized in periphery as well as central part of islets in WNIN-obese rats. Nestin was absent in leans rats and acute pancreatic induced expression of nestin in lean rats. Interestingly, induction of acute pancreatitis led to increased nestin-positive cells in WNIN-obese rats, as well lean rats. The immunohistochemical localization of nestin is depicted in Figure 1. Our observations indicate that higher pancreatic stress induces higher pancreatic stress (WNIN-obese + pancreatitis > WNIN-obese > lean + pancreatitis > lean). Our observation may play an important role in understanding the microenvironment requirement for differentiation of stem cells as activation of nestin-positive cells may play a functional role in pancreatic stress and inflammation.
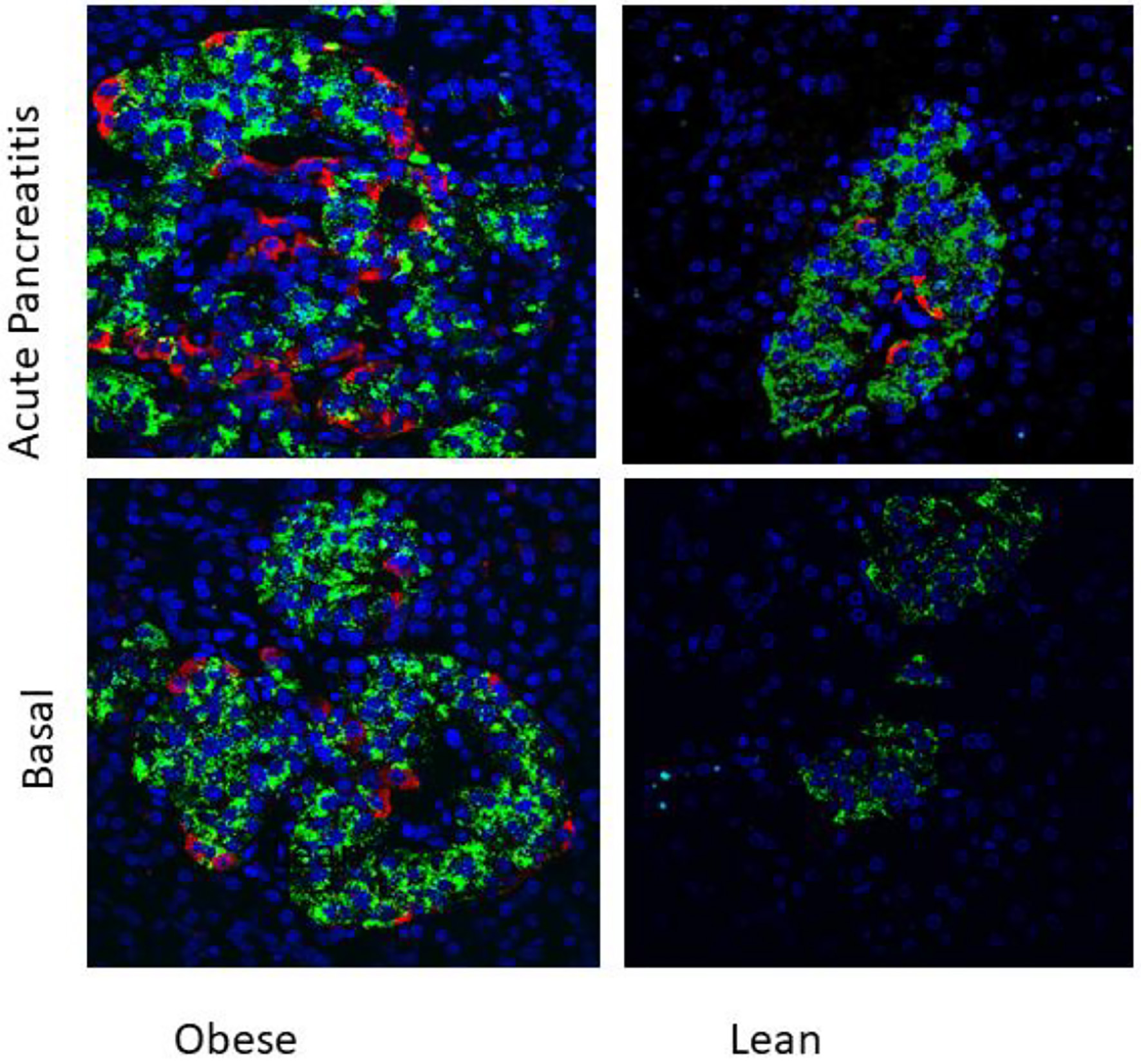 Click for large image | Figure 1. Immunolocalization of nestin (red) and insulin (green) in islets of 4 months old WNIN-obese and lean rats at basal conditions and after induction of acute pancreatitis (× 40). Presence of nestin-positive cells in islets of WNIN-obese rats at basal condition as well as when induced with acute pancreatitis. The induction of pancreatitis led to increased expression of nestin in these cells. Nestin-positive cells were absent in basal condition in lean rats but were present when induced with acute pancreatitis. The nestin-positive cells were localized in periphery as well as central part of islets in WNIN-obese rats. Higher pancreatic stress induced higher pancreatic stress (WNIN-obese + pancreatitis > WNIN-obese > lean + pancreatitis). Nestin was absent in islets of lean rats. |
The previously published studies have demonstrated increased inflammatory melieu in pancreas of WNIN-obese rats as demonstrated by altered cytoarchitecture of islets and expression of gene signature pertaining to inflammation. Moreover, induction of acute pancreatitis with intra-peritoneal administration of L-arginine led to increased severity of acute pancreatitis in WNIN-obese rats as compared lean rats. The WNIN-obese rats also demonstrated increased islet dysfunction and lean rats as compared to lean rats [21]. The mechanism of islet dysfunction is outlined in Figure 2.
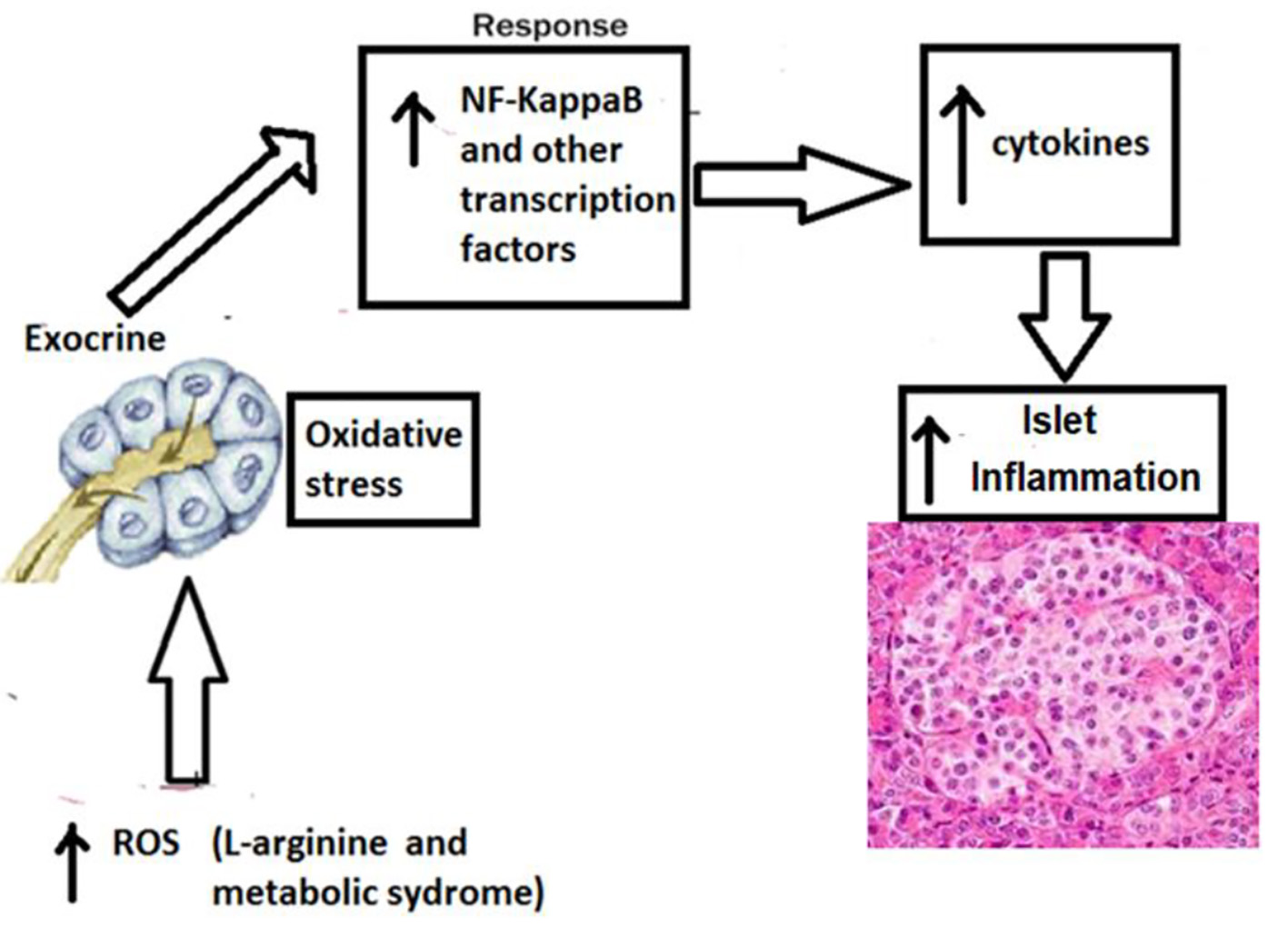 Click for large image | Figure 2. Mechanism of higher islet inflammation in WNIN-obese rats with acute pancreatitis. The participation of several confounding factors in the pancreatic milieu that collectively coprecipitates for a state of profound inflammation and oxidative stress in the pancreas (among mutant compared to lean/control) which gets worsened with age. These include hypertrophy, macrophage infiltration (CD11b/TNF-α/IL-6), apoptosis, β-cell vacuolation, HI, and stress markers (RL-77/HSP104/TBARS), all of which correlated well with indices for obesity (2 - 3 fold), IR (1.5 - 3 fold), and HI (2 - 3 fold). Further, supportive data were also obtained from in vitro studies using islet cell cultures amongst phenotypes. Taken together, these results advocate that oxidative stress and inflammation were the major precipitating factor to cause islet cell dysfunctions (in situ and in vitro) in these mutant rats compared to their lean littermates and parental control [2]. TNF-α: tumor necrosis factor-alpha; IL-6: interleukin-6; HI: hyperinsulinemia; IR: insulin resistance. |
The WNIN-obese rats are unique animal model for metabolic syndrome, as all metabolic defects observed in humans are seen in one model per se [22]. These animals are WNIN-obese, insulin-resistant, leptin-resistant, and have degenerative features associated with obesity such as retinal degeneration, high blood pressure, and accelerated aging with defective immune surveillance [8, 9, 22]. We have earlier reported a state of islet inflammation in these rats [8, 9]. Obesity-induced inflammation in pancreatic islet may trigger beta-cell apoptosis, leading to the progression of type 2 diabetes [23, 24]. Beta-cell regeneration in response to metabolic stress prevents development of type 2 diabetes [25]. Among many mechanism(s) under investigation, pancreatic stem cells may play an important role in beta-cell regeneration [26]. It is difficult to predict pancreatic stem cells from the knowledge of pancreatic development [27-30]. A study demonstrated that nestin is transiently expressed by acinar cells as well as insulin and glucagon cells of islets of mutant mice and demonstrated that nestin expression is regulated by glucagon signaling suggesting that the pattern of nestin staining is determined by the cellular environment and that nestin-positive cells located in the pancreatic primordium generate the cells of the endocrine and exocrine lineages [31]. We report for the first time the immunolocalization of nestin-positive cells in the pancreas of mice of different ages (3 days to 8 weeks) with reference to insulin and glucagon-positive cells. The heterogeneous localization of the nestin-positive cells observed may be of functional and developmental significance and suggest(s) that mice pancreatic tissue can be a potential source of progenitor cells. Nestin-positive cells from the pancreas can be isolated, proliferated and programmed to differentiate into insulin secreting cells under the appropriate microenvironment [17]. Further studies are required to understand the functional and development significance of nestin-positive cells in obesity and acute pancreatitis.
Conclusion
Nestin is one of the important markers of stem cells/progenitor cells in pancreas. The study reports localization of nestin in basal condition and acute pancreatitis condition in WNIN-obese rats, and in islets of lean rats with acute pancreatitis but absent in islets of lean rats at basal condition. Based on this observation, we hypothesize that pancreatic inflammation and pancreatic stress (obesity and acute pancreatitis) induce nestin-positive progenitor cells in islets.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
HS carried out the experiment. VV coordinated the overall study, including the project design and manuscript preparation.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Rodriguez-Hernandez H, Simental-Mendia LE, Rodriguez-Ramirez G, Reyes-Romero MA. Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol. 2013;2013:678159.
doi pubmed pmc - Kalashikam RR, Battula KK, Kirlampalli V, Friedman JM, Nappanveettil G. Obese locus in WNIN/obese rat maps on chromosome 5 upstream of leptin receptor. PLoS One. 2013;8(10):e77679.
doi pubmed pmc - Venkatesan V, Madhira SL, Malakapalli VM, Chalasani M, Shaik SN, Seshadri V, Kodavalla V, et al. Obesity, insulin resistance, and metabolic syndrome: a study in WNIN/Ob rats from a pancreatic perspective. Biomed Res Int. 2013;2013:617569.
doi pubmed pmc - Sinha JK, Ghosh S, Swain U, Giridharan NV, Raghunath M. Increased macromolecular damage due to oxidative stress in the neocortex and hippocampus of WNIN/Ob, a novel rat model of premature aging. Neuroscience. 2014;269:256-264.
doi pubmed - Reddy PY, Giridharan NV, Balakrishna N, Validandi V, Pullakhandam R, Reddy GB. Increased risk of cataract development in WNIN-obese rats due to accumulation of intralenticular sorbitol. IUBMB Life. 2013;65(5):472-478.
doi pubmed - Madhira SL, Nappanveethl G, Kodavalla V, Venkatesan V. Comparison of adipocyte-specific gene expression from WNIN/Ob mutant obese rats, lean control, and parental control. Mol Cell Biochem. 2011;357(1-2):217-225.
doi pubmed - Madhira SL, Challa SS, Chalasani M, Nappanveethl G, Bhonde RR, Ajumeera R, Venkatesan V. Promise(s) of mesenchymal stem cells as an in vitro model system to depict pre-diabetic/diabetic milieu in WNIN/GR-Ob mutant rats. PLoS One. 2012;7(10):e48061.
doi pubmed pmc - Singh H, Giridharan N, Bhonde R, Venkatesan V. Deriving at candidate genes of metabolic stress from pancreas of WNIN/GR-Ob mutant rats. Islets. 2013;5(4):133-138.
doi pubmed - Singh H, Ganneru S, Malakapalli V, Chalasani M, Nappanveettil G, Bhonde RR, Venkatesan V. Islet adaptation to obesity and insulin resistance in WNIN/GR-Ob rats. Islets. 2014;6(5-6):e998099.
doi pubmed pmc - Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem. 2010;58(8):721-730.
doi pubmed pmc - Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50(3):521-533.
doi pubmed - Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292(5520):1389-1394.
doi pubmed - Selander L, Edlund H. Nestin is expressed in mesenchymal and not epithelial cells of the developing mouse pancreas. Mech Dev. 2002;113(2):189-192.
doi - Piper K, Ball SG, Turnpenny LW, Brickwood S, Wilson DI, Hanley NA. Beta-cell differentiation during human development does not rely on nestin-positive precursors: implications for stem cell-derived replacement therapy. Diabetologia. 2002;45(7):1045-1047.
doi pubmed - Lechner A, Leech CA, Abraham EJ, Nolan AL, Habener JF. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cassette transporter. Biochem Biophys Res Commun. 2002;293(2):670-674.
doi - Tampaki EC, Nakopoulou L, Tampakis A, Kontzoglou K, Weber WP, Kouraklis G. Nestin involvement in tissue injury and cancer—a potential tumor marker? Cell Oncol (Dordr). 2014;37(5):305-315.
doi pubmed - Dorisetty RK, Kiran SG, Umrani MR, Boindala S, Bhonde RR, Venkatesan V. Immuolocalization of nestin in pancreatic tissue of mice at different ages. World J Gastroenterol. 2008;14(46):7112-7116.
doi pubmed pmc - Cigliola V, Thorel F, Chera S, Herrera PL. Stress-induced adaptive islet cell identity changes. Diabetes Obes Metab. 2016;(Suppl 1):87-96.
doi pubmed pmc - Venkatesan V, Gopurappilly R, Goteti SK, Dorisetty RK, Bhonde RR. Pancreatic progenitors: The shortest route to restore islet cell mass. Islets. 2011;3(6):295-301.
doi pubmed - Matsuda Y, Naito Z, Kawahara K, Nakazawa N, Korc M, Ishiwata T. Nestin is a novel target for suppressing pancreatic cancer cell migration, invasion and metastasis. Cancer Biol Ther. 2011;11(5):512-523.
doi pubmed pmc - Singh H, Ajumeera R, Malakapalli V, Chalasani M, Pothani S, Venkatesan V. WNIN mutant WNIN-Obese rats develop acute pancreatitis with the enhanced inflammatory milieu. Cellular and Molecular Medicine Research. 2017;1(1):20-31.
- Giridharan NV. Glucose & energy homeostasis: Lessons from animal studies. Indian J Med Res. 2018;148(5):659-669.
doi pubmed pmc - Singh H, Pragasam SJ, Venkatesan V. Emerging therapeutic targets for metabolic syndrome: lessons from animal models. Endocr Metab Immune Disord Drug Targets. 2019;19(4):481-489.
doi pubmed - Singh H, Venkatesan V. Treatment of 'Diabesity': beyond pharmacotherapy. Curr Drug Targets. 2018;19(14):1672-1682.
doi pubmed - Singh H, Parthasarathy V, Farouk M, Venkatesan V. Progenitor cells may aid successful islet compensation in metabolically healthy obese individuals. Med Hypotheses. 2016;86:97-99.
doi pubmed - Migliorini A, Bader E, Lickert H. Islet cell plasticity and regeneration. Mol Metab. 2014;3(3):268-274.
doi pubmed pmc - Houbracken I, Bouwens L. The quest for tissue stem cells in the pancreas and other organs, and their application in beta-cell replacement. Rev Diabet Stud. 2010;7(2):112-123.
doi pubmed pmc - Singh H. Islet Compensation in Metabolic Stress: Lessons from Animal Models. Curr Diabetes Rev. 2016;12(4):315-321.
doi pubmed - Singh H. Multi-omics approach to stem cell studies. Minerva Biotecno. 2017;29:169-173.
doi - Singh H. Bioinformatics toolbox for stem cell research and therapeutics. Stem Cell Therapy for Organ Failure. 2014. p. 31-37. Springer, New Delhi.
doi - Kedees MH, Guz Y, Vuguin PM, Vargas C, Cui L, Steiner DF, Charron MJ, et al. Nestin expression in pancreatic endocrine and exocrine cells of mice lacking glucagon signaling. Dev Dyn. 2007;236(4):1126-1133.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cellular and Molecular Medicine Research is published by Elmer Press Inc.



