| Cellular and Molecular Medicine Research, ISSN 2817-6359 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cell Mol Med Res and Elmer Press Inc |
| Journal website https://www.thecmmr.org |
Original Article
Volume 1, Number 2, December 2023, pages 56-60
Calcitonin Gene-Related Peptide Enhances the Expression of Signaling Molecules of the Wnt 7b/Beta-Catenin Pathway in Rat Type II Alveolar Epithelial Cells Under Hyperoxia
Shao-Hua Wanga, c, Yuan-Qing Chena, Long-Hui Lia, Li Lia, Hong-Xing Dangb, Feng Xub
aNeonatal Intensive Care Unit, Women and Children Health Institute Futian, University of South China, Shenzhen 518045, China
bPICU, Children’s Hospital of Chongqing Medical University; Ministry of Education Key Laboratory of Child Development and Disorders, Yu Zhong, Chongqing 400014, China
cCorresponding Author: Shao-Hua Wang, Neonatal Intensive Care Unit, Women and Children Health Institute Futian, University of South China, Shenzhen 518045, China
Manuscript submitted February 2, 2023, accepted April 6, 2023, published online November 7, 2023
Short title: Calcitonin Gene-Related Peptide and Hyperoxia
doi: https://doi.org/10.14740/cmmr7e
| Abstract | ▴Top |
Background: Hyperoxic lung injury is characterized by epithelial cell death and leukocyte infiltration/inflammation in the lung. Calcitonin gene-related peptide (CGRP) has been shown to improve survival of lung epithelial cells and reduce hyperoxic lung injury in rats. However, the mechanism of CGRP protective activity is not completely understood. The Wnt7b/β-catenin pathway plays an important role in lung development, repair and regeneration. This study therefore was designed to examine the regulatory role of CGRP in the Wnt7b/β-catenin pathway in type II alveolar epithelial cell (AEC II) under hyperoxia.
Methods: Rat neonates and AEC II from premature rats were exposed to hyperoxia in the absence or presence of CGRP, Wnt7b and β-catenin protein levels were measured by Western blot analysis, while T cell factor (TCF) and c-myc mRNA levels were determined by reverse transcription-polymerase chain reaction (RT-PCR).
Results: In the rat lung, both Wnt7b and β-catenin proteins were detectable, and hyperoxia significantly increased Wnt7b and β-catenin protein expression. AEC II, after exposure to hyperoxia, had significantly higher levels of Wnt7b and β-catenin proteins, and TCF and c-myc mRNAs, which was further enhanced by CGRP.
Conclusions: CGRP stimulates the expression of multiple molecules in the Wnt 7b/β-catenin pathway in AEC II under hyperoxia, which might be part of the mechanism by which CGRP protects the lung from hyperoxia-induced injury.
Keywords: Calcitonin gene-related peptide; Type II alveolar epithelial cell; Oxidative stress; Lung injury; Wnt7b; β-catenin
| Introduction | ▴Top |
Oxygen therapy constitutes a requisite part of respiratory support for preterm neonates, and also can be life-saving for adult patients under intensive care [1-3]. Excess supply of oxygen however, may lead to hyperoxia that can result in multi-organ injury including the lung [4-6]. Hyperoxic lung injury is characterized by epithelial cell death [7, 8], e.g., type II alveolar epithelial cell (AEC II) apoptosis is detected in rat lungs exposed to hyperoxia [8], and leukocyte infiltration/inflammation [9-11]. AEC II plays important roles in normal pulmonary function. They not only produce and secret pulmonary surfactant to maintain normal lung function but also involve in repair and regeneration after lung injury as AEC II, through differentiation, can give rise to type I AEC [12, 13].
A variety of bioactive molecules and complex signaling pathways are involved in lung development, repair and regeneration [14-17]. Neuropeptides/neurotransmitters can promote lung repair and regeneration by promoting lung epithelial cell growth, proliferation and differentiation [18-20]. In a previous study, we showed that calcitonin gene-related peptide (CGRP), a sensory neuropeptide, improved survival of AEC II cells and reduced oxidative stress-induced injury of the immature lung in rats [21]. However, the signaling pathways underlying the protective effects of CGRP on AEC II remain elusive. The Wnt signaling pathway, a complex signal transduction system, plays an important role in embryonic development, glucose homeostasis, cell proliferation, cell apoptosis, cell differentiation and migration [22]. To date, three Wnt signaling pathways have been recognized, which are 1) the canonical Wnt pathway, 2) the non-canonical planar cell polarity pathway, and 3) the non-canonical Wnt/calcium pathway. The canonical Wnt pathway regulates gene expression, controlling cell growth and death [22]. We hypothesized that the canonical Wnt pathway might be involved in CGRP protective activities in hyperoxic lung injury. This study was therefore conducted to examine the regulation of signaling molecules of the Wnt 7b/β-catenin pathway by CGRP in hyperoxia-treated AEC II isolated from immature rats.
| Materials and Methods | ▴Top |
DMEM/F12 medium and fetal bovine serum (FBS) were bought from Thermo Fisher Scientific China Co., Ltd (Shanghai, China). The total protein extraction kit was bought from Keygen Biotech. Co., Ltd (Nanjing, China). SDS-PAGE gels were from Boster Biological Engineering Co., Ltd (Wuhan, China). Anti-rat Wnt7b antibody, anti-rat β-catenin antibody, anti-β-actin antibody and horseradish peroxidase-conjugated anti-rabbit secondary antibody were obtained from Santa Cruz Biotechnology (Shanghai, China). CGRP was from AnaSpec Inc. (Fremont, CA, USA). PVDF membranes were from Amersham Phamacia Biotech (Beijing, China). Enhanced chemiluminescence chromogenic (ECL) system was bought from bioWorld (Dublin, OH, USA). RNA extraction kit was the product of Bioer Technology Co., Ltd (Hangzhou, China). The reverse transcription-polymerase chain reaction (RT-PCR) ReverTra Dash Kit was purchased from Toyobo Co., Ltd (Osaka, Japan). PCR primers were synthesized by Thermo Fisher Scientific China Co., Ltd.
Animal model
Use of animals and all animal procedures was approved by the Research Ethics Committee, Chongqing Medical University, in compliance with the Guidelines for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology, China. Timed-pregnant Sprague-Dawley rats were obtained from the Experimental Animal Center, Chongqing Medical University. Neonatal pups were immediately used after birth and treated as described elsewhere [21]. Briefly, two groups of pups (18 animals/group) were randomly selected with one group serving as controls that were maintained under room air with oxygen concentration controlled at 21±2%, while the other group of pups were placed in special Plexiglass chambers where oxygen concentration was kept between 93% and 97%. At days 3, 7 and 14, animals were sacrificed with intraperitoneal injection of pentobarbital (50 mg/kg). Afterwards, thoracic cavity was opened, and the lung was removed, rinsed with PBS, snap-frozen in liquid nitrogen and stored at -80 °C for protein extraction.
AEC II isolation, culture and treatment
AEC II was isolated from the fetus at gestational age of 21 days as described previously [23]. The identity of AEC II was determined by modified Papanicolaou staining and immunostaining using AEC II-specific antibodies as described elsewhere [23]. ACE II was cultured in DMEM/F12 medium containing 10% FBS at 37 °C under a humidified atmosphere containing 5% CO2. Cell treatment was done as follows: cells were seeded in six-well plates (5 × 105/well) and cultured for 24 h. Afterwards, spent medium was replaced with fresh medium and cells were divided into four groups: 1) air group, 2) air + CGRP (100 nM), 3) hyperoxia group, and 4) hyperoxia + CGRP (100 nM). Cells in groups 1 and 2 were cultured in a 37 °C incubator under a moisture atmosphere containing 5% CO2 for 24 h. Cells in groups 3 and 4 were placed in Plexiglas chambers filled with moisture carbogen (95% O2 and 5% CO2) and cultured at 37 °C for 24 h. Subsequently, cells were harvested for protein and mRNA assays.
Western blot analysis
Western blot analysis was performed to measure Wnt7b and β-catenin protein levels in rat lung tissue and AEC II. Protein samples were prepared as follows: 50 mg rat lung tissue was homogenized in 200 µL of lysis buffer (Keygen Biotech. Co., Ltd) or 2 million cultured AEC II lysed in 100 µL of lysis buffer. After centrifugation at 12,000 g for 20 min at 4 °C, the supernatant was harvested and the protein concentration was determined using the BCA protein assay kit (Bio-Rad Laboratories) as per the protocol provided by the manufacturer. Fifty micrograms of total protein for each sample was separated by SDS-PAGE and electrically transferred onto a PVDF membrane. The membrane was blocked with TBS-T buffer (10 mM Tris-HCl, pH 7.50, 9% NaCl and 0.05% Tween 20) containing 5% milk at room temperature for 1 h followed by incubation with the primary antibody at 4 °C overnight. All primary antibodies were used at dilutions of 1:500. After three washes (5 min/wash) with TBS-T buffer, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1:3,000 dilution) at room temperature for 1 h. Afterwards, the membrane was washed three times with TBS-T buffer and the target protein was detected with an ECL chemiluminescence kit. ChemiDocXRS image acquisition system (Bio-Rad Laboratories, Shanghai, China) was used for blot imaging. The Quantity One 4.5.0 software (Bio-Rad Laboratories) was used for densitometric analysis of protein bands, and semi-quantification of each target protein was done after normalization against β-actin.
RT-PCR
Total RNA from AEC II was extracted using an RNA TRIzol kit (Bioer Technology Co., Ltd) according to the manufacturer’s instructions and quantified with spectrophotometry. Reverse transcription was done using 0.5 µg total RNA following the kit instructions. PCR was done using 1 µL of RT product and run as follows: 95 °C for 3 min followed by 30 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 60 s. The PCR product was analyzed by 1.5% agarose gel electrophoresis. Semi-quantification of PCR products after normalized against β-actin was performed.
The sequences of primers were as follows: c-my: forward, 5’-GGAGAAACGAGCTGAAGCGTAG-3’, reverse, 5’-CAGCCAAGGTTGTGAGGTTAGG-3’ (339 bp); TCF: forward, 5’-ATGACACGGATGACGATGGG-3’, reverse, 5’-GGACAGGTGGGACTGGTTGAG-3’ (221 bp); β-actin, forward, 5’-TCACCCACACTGTGCCCATCTATGA-3’, reverse, 5’-CATCGGAACCGCTCATTGCCGATAG-3’ (295 bp).
| Results | ▴Top |
Five pups died in the hyperoxic group while two died in the control group at the end of study period. Western blot analysis revealed that both Wnt7b and β-catenin proteins were detectable in the lung, and levels of both proteins did not change at different time points in control group (Fig. 1a). However, at all time points, both Wnt7b and β-catenin protein levels in hyperoxic group were significantly higher compared with respective controls. Within the hyperoxic group, both Wnt7b and β-catenin protein expressions were significantly increased at day 7 compared with those at days 3 and 14 (both P < 0.05).
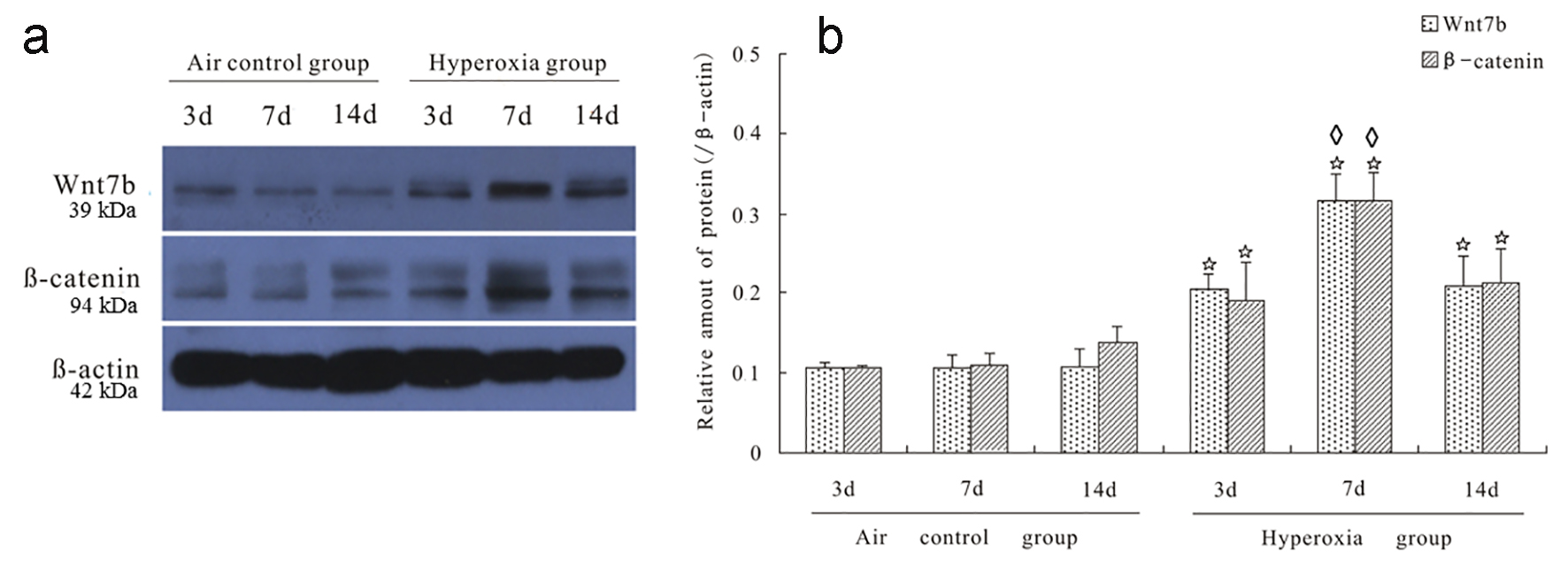 Click for large image | Figure 1. Hyperoxia increased the expression of Wnt7b and β-catenin proteins in rat lungs. Panel a shows a representative Western blot for Wnt7b and β-catenin protein analysis in rat lungs. Under normoxia, Wnt7b and β-catenin proteins maintained at a consistent level at all time points observed (panels a and b). In contrast, hyperoxia increased the expression of Wnt7b and β-catenin proteins in rat lungs at all time points (panels a and b). *P < 0.05 compared with their respective controls at the same time point. In the hyperoxic group, at day 7, both Wnt7b and β-catenin protein levels were significantly higher than their respective levels at other two time points (◊P < 0.05). |
There was no significant difference in Wnt7b and β-catenin protein levels between AEC II under normoxia and normoxia plus CGRP (Fig. 2). However, hyperoxia induced a markedly increased expression of Wnt7b and β-catenin proteins, which was significantly enhanced by CGRP (Fig. 2). We further measured the mRNA expression of TCF and c-myc in AEC II, and the results showed that TCF and c-myc mRNA levels were similar between AEC II under normoxia and normoxia plus CGRP. In contrast, hyperoxia resulted in significantly elevated TCF and c-myc mRNA expression, which was further boosted by CGRP treatment (Fig. 3).
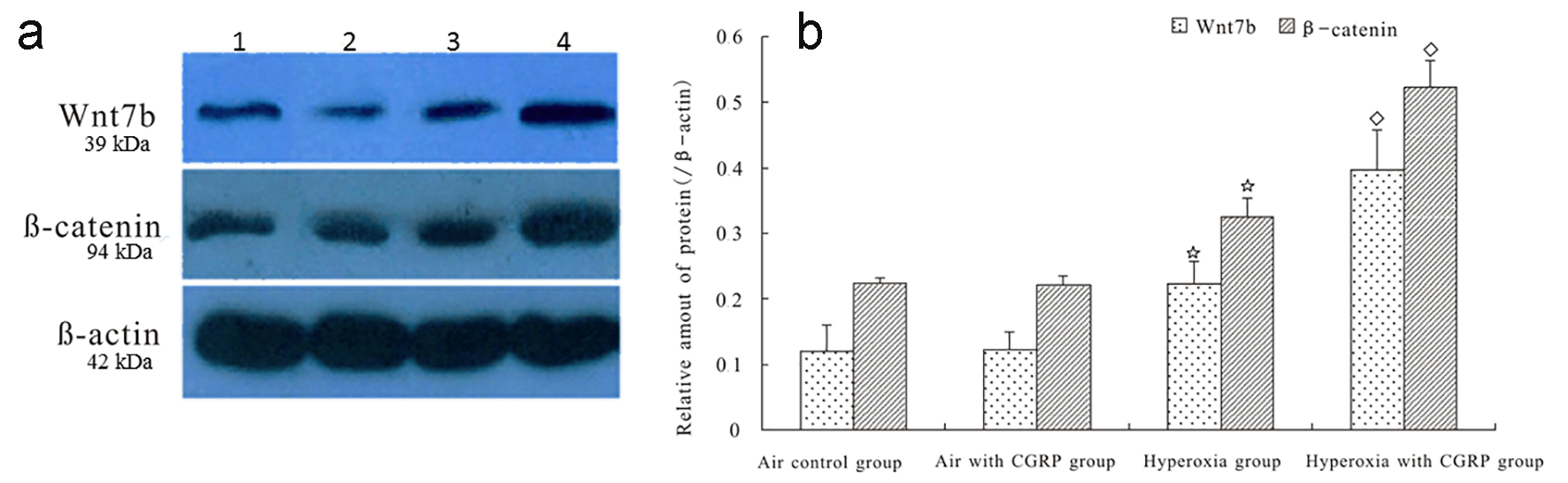 Click for large image | Figure 2. CGRP enhanced hyperoxia-induced expression of Wnt7b and β-catenin proteins in AEC II. There was no significant difference in Wnt7b and β-catenin protein levels between AEC II under normoxia and normoxia plus CGRP (panels a and b). However, hyperoxia induced a markedly increased expression of Wnt7b and β-catenin proteins (*P < 0.05), which was significantly enhanced by CGRP (◊P < 0.05, panels a and b). 1 = Air control; 2 = Air with CGRP; 3 = Hyperoxia; and 4 = Hyperoxia with CGRP. |
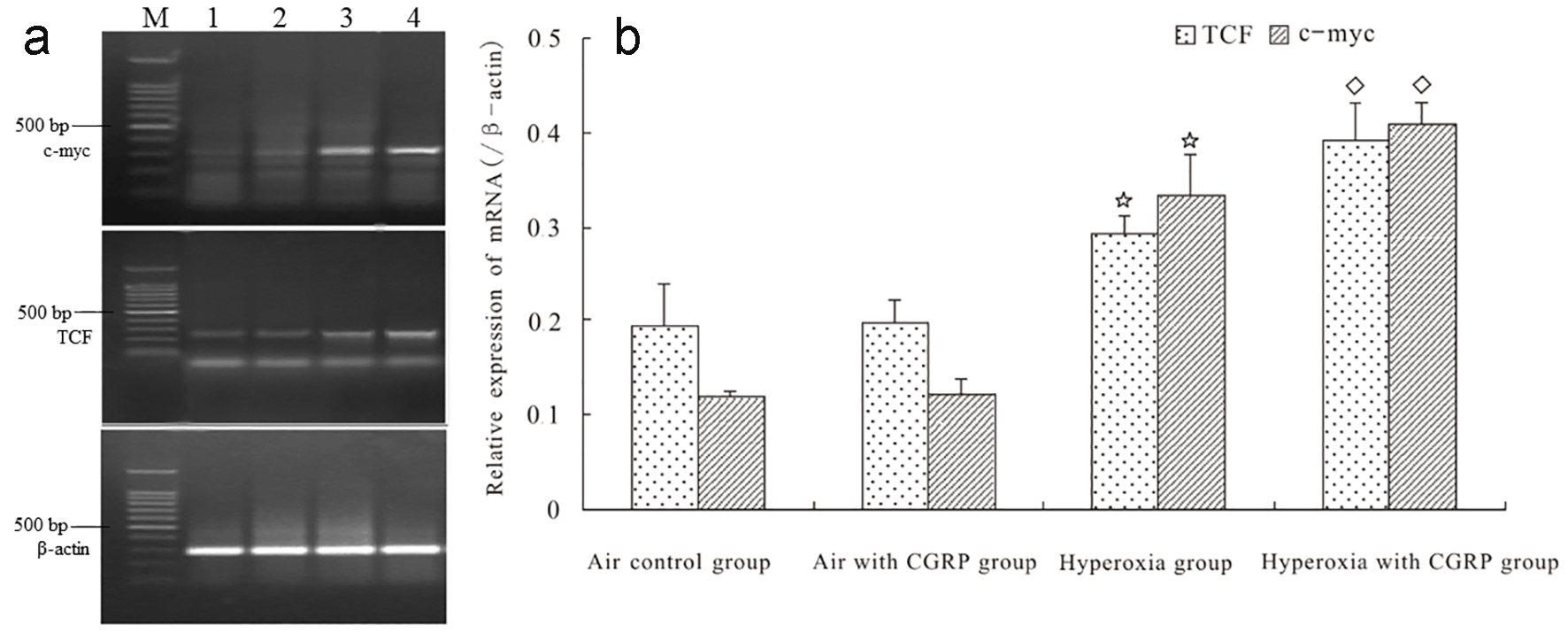 Click for large image | Figure 3. CGRP enhanced hyperoxia-induced mRNA expression of TCF and c-myc in AEC II. TCF and c-myc mRNA levels were similar between AEC II under normoxia and normoxia plus CGRP (panels a and b). In contrast, hyperoxia resulted in significantly elevated TCF and c-myc mRNA expression (*P < 0.05), which was further boosted by CGRP treatment (◊P < 0.05, panels a and b). M = DNA size marker; 1 = Air control; 2 = Air with CGRP; 3 = Hyperoxia; and 4 = Hyperoxia with CGRP. |
| Discussion | ▴Top |
Therapeutic use of oxygen is necessary for patients having critical pulmonary and cardiac conditions especially for premature neonates [1-3]. Over-exposure to high concentration of oxygen-caused lung injury still occurs in current clinical practice but effective therapy for such injury is limited. In an effort to seek biological molecules for effective treatment of hyperoxic lung injury, we previously discovered that CGRP had a protective role in hyperoxia-induced lung injury in rats [21]. In this study, we aimed at delineating the role of CGRP in regulating the expression of signaling molecules of the Wnt 7b/β-catenin pathway. We reported here the following findings: 1) hyperoxia increased expression of Wnt7b and β-catenin proteins in rat lungs at day 7 post hyperoxia exposure, which was diminished at day 14; 2) exposure to hyperoxia for 24 h augmented the expression of Wnt 7b and β-catenin proteins in AEC II isolated from premature rats, which was further enhanced by CGRP treatment; and 3) exposure to hyperoxia for 24 h increased the mRNA levels of TCF and c-myc in AEC II from premature rats, which was also enhanced by CGRP.
The canonical Wnt pathway, also termed Wnt/β-catenin pathway, consists of several activators, i.e., Wnt1, Wnt3, Wnt3a, Wnt7a, Wnt7b and Wnt8 [14]. These activators bind to the Frizzled receptor on the cell membrane and activate the cytoplasmic phosphoprotein Dishevelled (Dsh/Dvl) [24], leading to the accumulation and stabilization of β-catenin in the cytoplasm. Subsequently, β-catenin is translocated into nuclei where it forms an active transcription complex with members of the TCF/lymphoid enhancer factor (TCF/LEF), initiating the expression of target genes such as surviving, c-myc and cyclin D1, thereby regulating cellular processes and functions [25, 26].
It is known that the canonical Wnt pathway is involved in lung development, repair and regeneration. Okubo and Hogan revealed that the Wnt pathway was hyperactive in mouse embryonic lungs [27]. Mucenski et al reported that knockout of β-catenin, a central molecule of the canonical Wnt pathway in mice resulted in defects of peripheral airway development in the lung, and pups died at birth because of respiratory failure [28]. Of all canonical Wnt pathway activators, Wnt 7b is the only one whose expression is restricted to the pulmonary epithelium [29]. It has been shown that Wnt 7b is strictly controlled by the lung-restricted transcription factor TTF-1 that is abundant in AEC II [30]. These data suggest Wnt 7b plays an important role in regulating AEC II functions. Based on these observations, we focused on determining whether the Wnt 7b/β-catenin pathway was altered by CGRP treatment in AEC II. First, we observed that Wnt 7b and β-catenin proteins were expressed in rat lungs, which, after exposure to hyperoxia for 7 days, was elevated. This elevation is likely a self-protective response of the body to the detrimental effects of hyperoxia. Indeed, this elevation was eventually diminished after the hyperoxic condition prolonged.
Next, we examined the effects of CGRP on the expression of signaling molecules, i.e., Wnt 7b, β-catenin, c-myc and TCF in ACE II treated with hyperoxia. In agreement with the finding in animal experiment, hyperoxia led to increased expression of Wnt 7b and β-catenin proteins in ACE II, which was further enhanced by CGRP. Additionally, using RT-PCR, we showed that mRNA levels of c-myc and TCF were elevated in ACE II after exposure to hyperoxia, which was also intensified by CGRP. Flozak et al reported that catenin/TCF signaling was activated during lung injury, which is in line with our findings, and activation of catenin/TCF promoted the survival of AECs [16]. C-myc acts primarily as a cell growth factor, and therefore elevation of c-myc expression under CGRP treatment might exert protective effects on AEC II.
In conclusion, CGRP stimulates the expression of multiple molecules in the Wnt 7b/β-catenin pathway in AEC II under hyperoxia, which might be part of the mechanism by which CGRP protects the lung from hyperoxia-induced injury. Limits of this study include: 1) CGRP impact on gene expression in the Wnt 7b/β-catenin pathway in the rat lung under hyperoxia was not examined, 2) whether inhibition of the Wnt 7b/β-catenin pathway would abolish CGRP protective effects in the rat lung under hyperoxia was not investigated, and 3) whether CGRP could prevent AEC II from hyperoxia-induced apoptosis was not explored.
Conflicts of Interest
The authors declare that we have no conflicts of interest.
| References | ▴Top |
- Walsh BK, Brooks TM, Grenier BM. Oxygen therapy in the neonatal care environment. Respir Care. 2009;54(9):1193-1202.
pubmed - Bateman NT, Leach RM. ABC of oxygen. Acute oxygen therapy. BMJ. 1998;317(7161):798-801.
doi - Parke RL, Eastwood GM, McGuinness SP. Oxygen therapy in non-intubated adult intensive care patients: a point prevalence study. Crit Care Resusc. 2013;15(4):287-293.
pubmed - Perrone S, Bracciali C, Di Virgilio N, Buonocore G. Oxygen Use in Neonatal Care: A Two-edged Sword. Front Pediatr. 2016;4:143.
doi pubmed pmc - Mach WJ, Thimmesch AR, Pierce JT, Pierce JD. Consequences of hyperoxia and the toxicity of oxygen in the lung. Nurs Res Pract. 2011;2011:260482.
doi - Davis WB, Rennard SI, Bitterman PB, Crystal RG. Pulmonary oxygen toxicity. Early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med. 1983;309(15):878-883.
doi pubmed - Pagano A, Barazzone-Argiroffo C. Alveolar cell death in hyperoxia-induced lung injury. Ann N Y Acad Sci. 2003;1010:405-416.
doi pubmed - Buckley S, Barsky L, Driscoll B, Weinberg K, Anderson KD, Warburton D. Apoptosis and DNA damage in type 2 alveolar epithelial cells cultured from hyperoxic rats. Am J Physiol. 1998;274(5 Pt 1):L714-720.
pubmed - Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58(1):123-141.
doi pubmed pmc - Lagishetty V, Parthasarathy PT, Phillips O, Fukumoto J, Cho Y, Fukumoto I, Bao H, et al. Dysregulation of CLOCK gene expression in hyperoxia-induced lung injury. Am J Physiol Cell Physiol. 2014;306(11):C999-C1007.
doi pubmed pmc - Weichelt U, Cay R, Schmitz T, Strauss E, Sifringer M, Buhrer C, Endesfelder S. Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur Respir J. 2013;41(4):966-973.
doi pubmed - Ward HE, Nicholas TE. Alveolar type I and type II cells. Aust N Z J Med. 1984;14(5 Suppl 3):731-734.
doi pubmed - Castranova V, Rabovsky J, Tucker JH, Miles PR. The alveolar type II epithelial cell: a multifunctional pneumocyte. Toxicol Appl Pharmacol. 1988;93(3):472-483.
doi - Pongracz JE, Stockley RA. Wnt signalling in lung development and diseases. Respir Res. 2006;7:15.
doi pubmed pmc - Rajagopal J, Carroll TJ, Guseh JS, Bores SA, Blank LJ, Anderson WJ, Yu J, et al. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135(9):1625-1634.
doi pubmed pmc - Flozak AS, Lam AP, Russell S, Jain M, Peled ON, Sheppard KA, Beri R, et al. Beta-catenin/T-cell factor signaling is activated during lung injury and promotes the survival and migration of alveolar epithelial cells. J Biol Chem. 2010;285(5):3157-3167.
doi pubmed pmc - Zaher TE, Miller EJ, Morrow DM, Javdan M, Mantell LL. Hyperoxia-induced signal transduction pathways in pulmonary epithelial cells. Free Radic Biol Med. 2007;42(7):897-908.
doi pubmed pmc - Wang W, Jia L, Wang T, Sun W, Wu S, Wang X. Endogenous calcitonin gene-related peptide protects human alveolar epithelial cells through protein kinase Cepsilon and heat shock protein. J Biol Chem. 2005;280(21):20325-20330.
doi pubmed - Kawanami Y, Morimoto Y, Kim H, Nakamura T, Machida K, Kido T, Asonuma E, et al. Calcitonin gene-related peptide stimulates proliferation of alveolar epithelial cells. Respir Res. 2009;10:8.
doi pubmed pmc - Li WJ, Wang TK. Calcitonin gene-related peptide inhibits interleukin-1beta-induced interleukin-8 secretion in human type II alveolar epithelial cells. Acta Pharmacol Sin. 2006;27(10):1340-1345.
doi pubmed - Dang H, Yang L, Wang S, Fang F, Xu F. Calcitonin gene-related peptide ameliorates hyperoxia-induced lung injury in neonatal rats. Tohoku J Exp Med. 2012;227(2):129-138.
doi pubmed - Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781-810.
doi pubmed - Chen J, Chen Z, Narasaraju T, Jin N, Liu L. Isolation of highly pure alveolar epithelial type I and type II cells from rat lungs. Lab Invest. 2004;84(6):727-735.
doi pubmed - Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22(5):717-727.
doi pubmed - Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281(32):22429-22433.
doi pubmed - MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9-26.
doi pubmed pmc - Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3(3):11.
doi pubmed pmc - Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, et al. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278(41):40231-40238.
doi pubmed - Weidenfeld J, Shu W, Zhang L, Millar SE, Morrisey EE. The WNT7b promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. J Biol Chem. 2002;277(23):21061-21070.
doi pubmed - Minoo P, Hamdan H, Bu D, Warburton D, Stepanik P, deLemos R. TTF-1 regulates lung epithelial morphogenesis. Dev Biol. 1995;172(2):694-698.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cellular and Molecular Medicine Research is published by Elmer Press Inc.



